

Theory Behind Determining Molar Enthalpy of Solution
Chemistry thermodynamics calculator free#
No ads = no money for us = no free stuff for you! (ii) ΔH is positive if energy (heat) is absorbed ( endothermic).
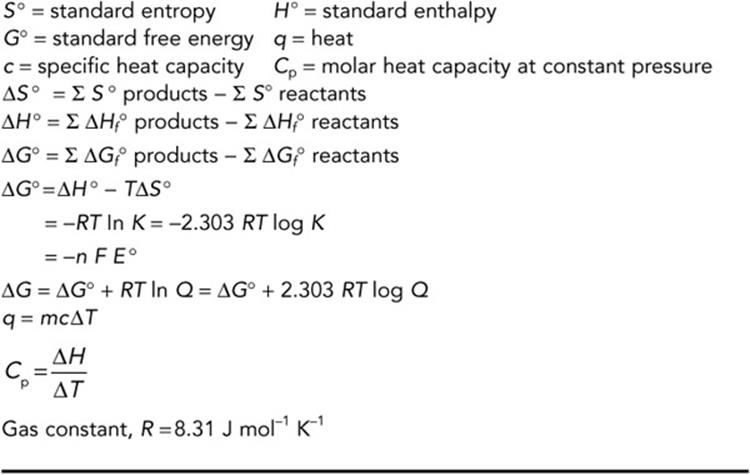
(i) ΔH is negative if energy (heat) is released ( exothermic). Note: You must include the sign for ΔH soln (either + or −) Q = amount of energy (heat) released or absorbed

ΔH soln = molar enthalpy (heat) of solution Step 3: Calculate mount of energy (heat) released or absorbed per mole of solute (ΔH soln) Q = amount of energy released or absorbed Step 1: Calculate the amount of energy released or absorbed (q) ⚛ To calculate the molar enthalpy of solution (molar heat of solution) using experimental data: ⚛ Enthalpy (heat) of solution can be determined in the laboratory by measuring the temperature change of the solvent when solute is added. ⚛ If heat is released when the solute dissolves, temperature of solution increases, reaction is exothermic, and ΔH is negative (ΔH 0). ⚛ Molar heat of solution (molar enthalpy of solution) has the units (2) J mol -1 or kJ mol -1 ⚛ Heat of solution (enthalpy of solution) has the symbol (1) ΔH soln ⚛ Molar heat of solution, or, molar enthalpy of solution, is the energy released or absorbed per mole of solute being dissolved in solvent. ⚛ Heat of solution, or, enthalpy of solution, is the energy released or absorbed when the solute dissolves in the solvent. ⚛ A solute dissolves in excess solvent to form a solution:


 0 kommentar(er)
0 kommentar(er)
